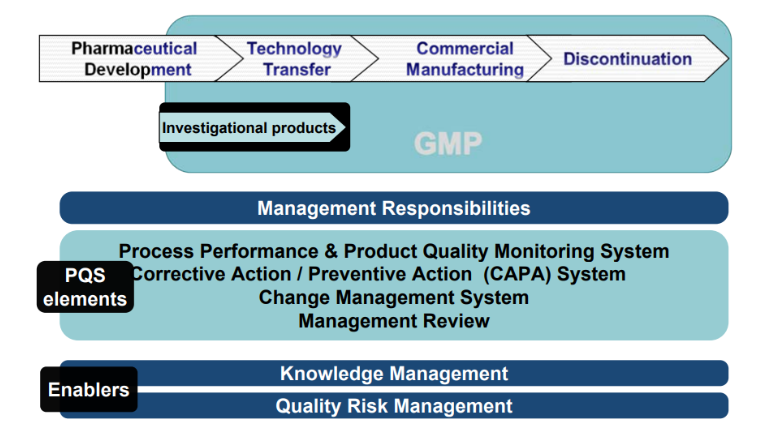
QMS and GMP
Documentation
Quality management, defining the overall policy of the organization towards quality where we help to build our client a robust quality management system to meet regulatory expectations, the need of the organization, and interested parties; i.e. customers. Thereby we have to achieve the PQMS goal for the organization:

- Achieve Product Realization: We guide to establish, implement and maintain a system that allows the delivery of products with quality attributes appropriate to meet the needs of patients and other stakeholders.
- Establish and Maintain a State of Control: We guide to develop & use effective monitoring & control systems for process performance & product quality, thereby assuring continued suitability and capability of processes using a risk-based approach and road mapping to implement the same.
Facilitate Continual Improvement: We help to identify and implement appropriate product quality improvements, process improvements, variability reduction, innovation and quality system enhancements, thereby increasing the ability to fulfill quality needs consistently.
QMS Support Services
- Preparation of risk assessment Document for process, facility, equipment, cleaning, cross-contamination, and as required
- Product/ Process development report
- Writing BMR, MFR, equipment /instrument list
- Preparation and revision of Site Master File
- Preparation and revision of the Validation Master Plan
- Preparation of quality manual/laboratory management manual and Quality Policy
- Product Quality Failure investigation report writing
- Writing Audit and inspection compliance report
- Assessment reports for change / deviation management
- Excel sheet/calculator/reagent validation Documents
- Writing / Review and revision of SOPS of QA, QC, Store, Supply chain, Production, R&D, and Corporate QA SOPs
- Preparation and review of quality specification/test protocols for RM/PM and finished products
- Preparation /review of annual product review and suggest actions to need base optimization of process
- Review of documents and approvals from regulatory agencies to identify gaps and non-compliances.